
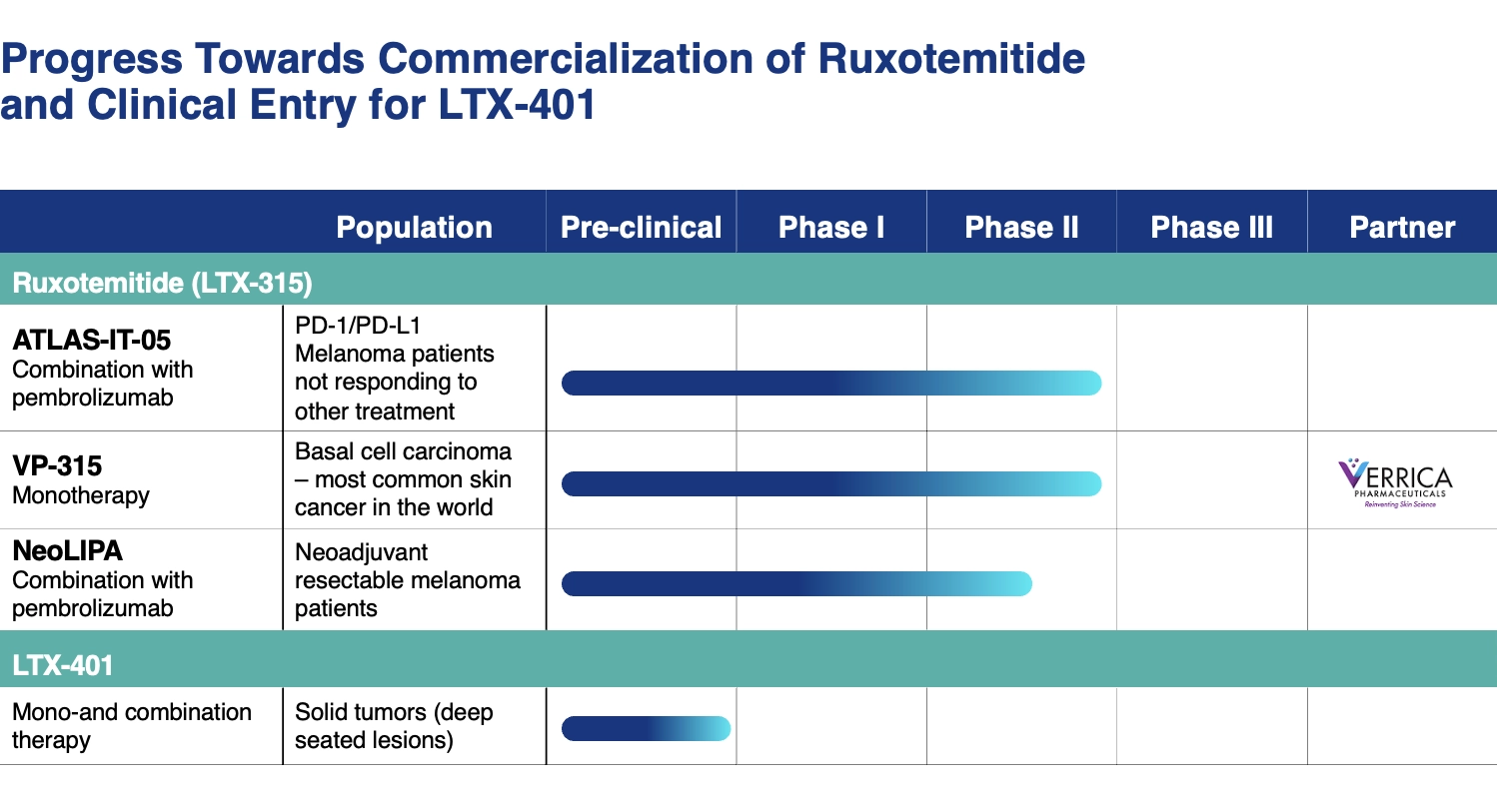
Our lead candidate, LTX-315, has been studied in several completed Phase I/II studies that in total enrolled almost 100 patients with various solid cancer types (e.g. melanoma, breast cancer, soft tissue sarcoma and head and neck cancer).
NeoLIPA
An investigator-initiated Phase 2 study with ruxotemitide is currently underway at the Oslo University Hospital. This study is exploring neo-adjuvant ruxotemitide (administered before surgery) in combination with standard of care pembrolizumab (KEYTRUDAⓇ) in patients with resectable melanoma. The objective of this study is to demonstrate that ruxotemtidie improves outcomes in these patients and prevents disease recurrence.
Verrica Pharmaceuticals sponsored Phase II Study in Basal Cell Carcinoma
Verrica has generated impressive Phase 2 data in basal cell carcinoma with ruxotemitide as a monotherapy. They have reported a 51% complete histologic clearance rate, and calculated objective response rate (ORR) in 97% of the patients with significant reduction of tumor size.
Additionally, Verrica has demonstrated that ruxotemitide reprograms the tumor microenvironment, with patients biopsies showing significant increases in CD4+, CD8+ T cells, and B-cells, and decrease of immune suppressive cells (Tregs and M2 machrophages) indicating strong recruitment of effector immune populations into the tumor.
